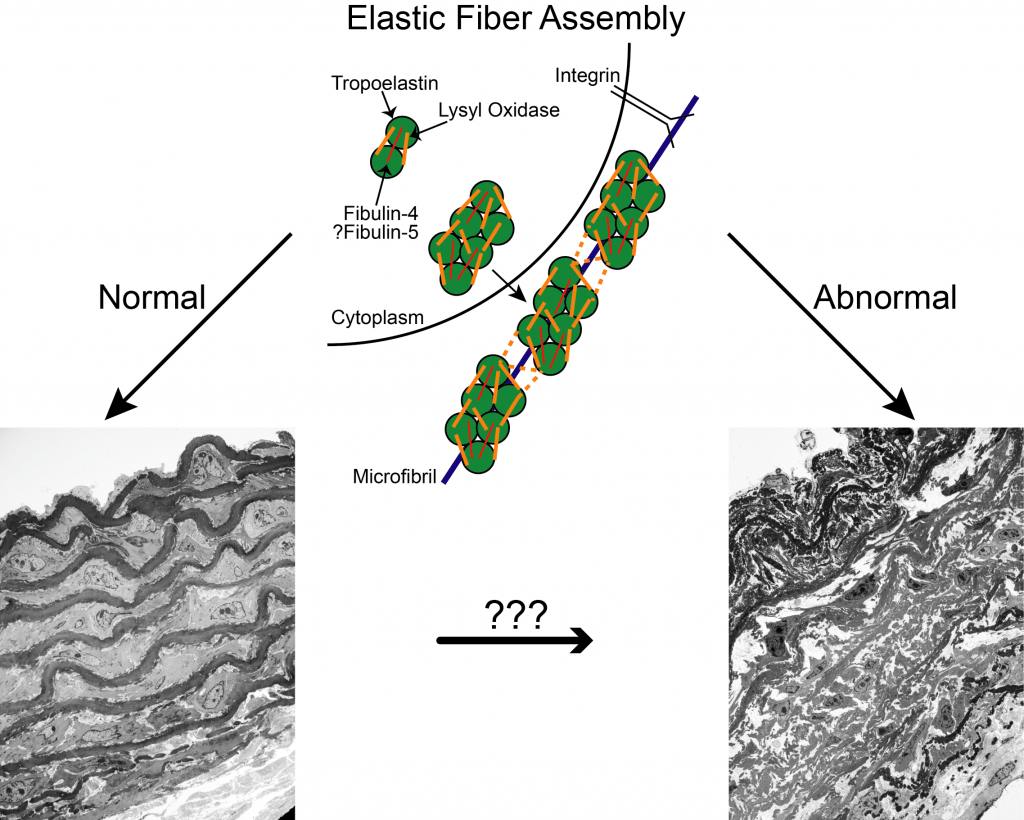
An important protein necessary for the development of a closed circulatory system in vertebrates is the extracellular matrix protein elastin. Elastin, the main component of vascular elastic fibers, confers elasticity to large arteries allowing them to store energy during systole and to release it during diastole, which reduces the load on the heart and dampens the pulse pressure generated by each heartbeat. While elastin is the main component of elastic fibers, several other proteins are necessary for their assembly, including fibulin-4, fibulin-5 and lysyl oxidase. Lysyl oxidase is known to mediate crosslinking of elastin molecules; the roles fibulin-4 and fibulin-5 play in elastic fiber assembly are less well understood however. Mutations in each of these molecules have been identified in humans and are associated with diseases including cutis laxa and familial thoracic aortic aneurysms and dissection. In our lab, we utilize mouse models that are either deficient or that carry disease-causing mutations in these genes to understand their role in the process of elastic fiber assembly. In addition to understanding normal vascular development, these studies will help us identify potentially targetable pathways to treat the consequences of abnormal elastic fiber assembly, including aneurysms and hypertension.
Current ongoing projects in the lab include:
- Examining the interaction between lysyl oxidase and fibulin-4 in the process of elastic fiber assembly in vivo.
- Identifying the differential contribution of fibulin-4 and fibulin-5 to elastic fiber assembly along the arterial tree, particularly with respect to small vs large arteries.
- Determining the cell-specific contribution of lysyl oxidase to elastic fiber formation.
- Understanding the role of insulin-like growth factor binding protein 2 (IGFBP2), which was highly upregulated in fibulin-4 mutant aorta, in abnormal vessel wall development.